
Chemistry, 05.07.2019 19:10, daniiltemkin20
The equilibrium constant for the reaction agbr(s) picture ag+(aq) + br− (aq) is the solubility product constant, ksp = 7.7 × 10−13 at 25°c. calculate δg for the reaction when [ag+] = 1.0 × 10−2 m and [br-] = 1.0 × 10−3 m. is the reaction spontaneous or nonspontaneous at these concentrations?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
Do you know the correct answer?
The equilibrium constant for the reaction agbr(s) picture ag+(aq) + br− (aq) is the solubility produ...
Questions in other subjects:

History, 21.08.2019 19:30




Mathematics, 21.08.2019 19:30

Geography, 21.08.2019 19:30

Mathematics, 21.08.2019 19:30

Biology, 21.08.2019 19:30



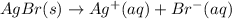
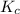 :
:![K_c=\frac{[Ag^+][Br^-]}{[AgCl]}=\frac{[Ag^+][Br^-]}{1}=[Ag^+][Br^-]](/tpl/images/0055/1226/d9269.png)
![K_{sp}=[Ag^+][Br^-]=K_c=7.7\times 10^{-13}](/tpl/images/0055/1226/964b9.png)
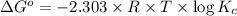
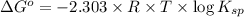
![\Delta G^o=-2.303\times 8.314 J/K mol\times 298 K\times \log[7.7\times 10^{-13}]](/tpl/images/0055/1226/37a54.png)
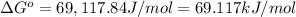
![[Ag^+] = 1.0\times 10^{-2} M](/tpl/images/0055/1226/88952.png) and
and ![[Br^-] = 1.0\times 10^{-3} M](/tpl/images/0055/1226/50b47.png)
![Q=[Ag^+][Br^-]=1.0\times 10^{-2} M\times 1.0\times 10^{-3} M=1.0\times 10^{-5}](/tpl/images/0055/1226/4f48a.png)
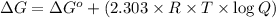
![\Delta G=69.117 kJ/mol+(2.303\times 8.314 Joule/mol K\times 298 K\times \log[1.0\times 10^{-5}])](/tpl/images/0055/1226/947e2.png)
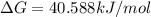
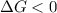 .For reaction to non spontaneous reaction:
.For reaction to non spontaneous reaction: 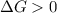 .
.



