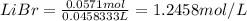Chemistry, 05.07.2019 05:20, claftonaustin846
Write the balanced chemical equation for the following acid and base reaction. (use the lowest possible whole number coefficients. include states-of-matter under the given conditions in your answer.)
hbr(aq) + lioh(aq) →
a) using the balanced reaction above, calculate the amount of 0.0024 m lioh that would neutralize 22 ml of 0.0026 m hbr.
b)how many moles of salt are produced in the reaction?
c)what is the molar concentration of the salt after the reaction is complete?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
Do you know the correct answer?
Write the balanced chemical equation for the following acid and base reaction. (use the lowest possi...
Questions in other subjects:

Spanish, 14.02.2021 21:10

Mathematics, 14.02.2021 21:10

Mathematics, 14.02.2021 21:10

Mathematics, 14.02.2021 21:10

Mathematics, 14.02.2021 21:10


English, 14.02.2021 21:10


Mathematics, 14.02.2021 21:10

Mathematics, 14.02.2021 21:10


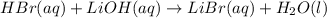
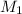 = 0.0024 M
= 0.0024 M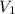
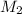 = 0.0026 M
= 0.0026 M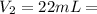
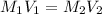
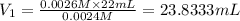
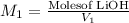
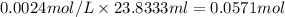
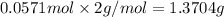
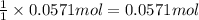 of LiBr
of LiBr