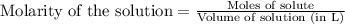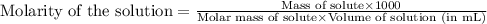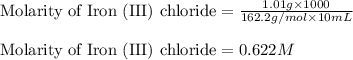Chemistry, 03.07.2019 21:30, lizzyhearts
7. suppose 1.01 g of iron (iii) chloride is placed in a 10.00-ml volumetric flask with a bit of water in it. the flask is shaken to dissolve the solid and the flask is then filled to the mark. what is the molarity of the final solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
Do you know the correct answer?
7. suppose 1.01 g of iron (iii) chloride is placed in a 10.00-ml volumetric flask with a bit of wate...
Questions in other subjects:



English, 27.07.2020 22:01




Arts, 27.07.2020 22:01


Mathematics, 27.07.2020 22:01