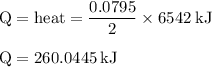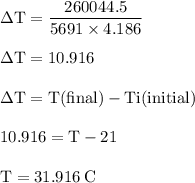Chemistry, 03.07.2019 21:20, tannerweberp5r8sg
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 6.200 g c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
Do you know the correct answer?
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 6.200 g c...
Questions in other subjects:

Mathematics, 04.07.2019 19:30

History, 04.07.2019 19:30




English, 04.07.2019 19:30


Mathematics, 04.07.2019 19:30