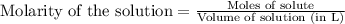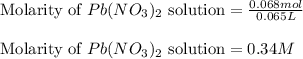Chemistry, 03.07.2019 20:30, ronaldhernandez598
Asolution of nacl(aq) is added slowly to a solution of lead nitrate, pb(no3)2(aq) , until no further precipitation occurs. the precipitate is collected by filtration, dried, and weighed. a total of 18.86 g pbcl2(s) is obtained from 200.0 ml of the original solution. calculate the molarity of the pb(no3)2(aq) solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, jescanarias22
What’s the scientific notation for the number 6,840,000,000
Answers: 1


Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
Do you know the correct answer?
Asolution of nacl(aq) is added slowly to a solution of lead nitrate, pb(no3)2(aq) , until no further...
Questions in other subjects:


Social Studies, 02.02.2020 23:02


English, 02.02.2020 23:02





Chemistry, 02.02.2020 23:02


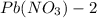 solution is 0.34 M.
solution is 0.34 M.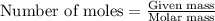
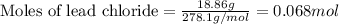

 of lead nitrate
of lead nitrate