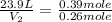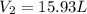Chemistry, 02.07.2019 19:20, azertyqwerty123
Asample of gas contains 0.1300 mol of n2(g) and 0.2600 mol of o2(g) and occupies a volume of 23.9 l. the following reaction takes place: n2(g) + 2o2(g)2no2(g) calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, Thomas7785
Two things that biomedical has invented or innovated
Answers: 1

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2

Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1
Do you know the correct answer?
Asample of gas contains 0.1300 mol of n2(g) and 0.2600 mol of o2(g) and occupies a volume of 23.9 l....
Questions in other subjects:

Mathematics, 24.09.2020 22:01


Mathematics, 24.09.2020 22:01

History, 24.09.2020 22:01


Mathematics, 24.09.2020 22:01





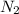 = 0.13 mole
= 0.13 mole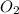 = 0.26 mole
= 0.26 mole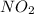 gas.
gas.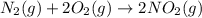
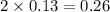 moles of
moles of 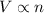
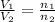
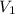 = initial volume of gas = 23.9 L
= initial volume of gas = 23.9 L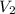 = final volume of gas = ?
= final volume of gas = ?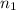 = initial moles of gas = 0.13 + 0.26 = 0.39 mole
= initial moles of gas = 0.13 + 0.26 = 0.39 mole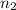 = final moles of gas = 0.26 mole
= final moles of gas = 0.26 mole