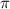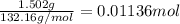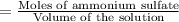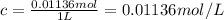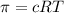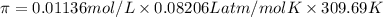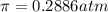Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2
Do you know the correct answer?
Calculate the osmotic pressure of a solution containing 1.502 g of (nh4)2so4 in 1 l at 36.54 degrees...
Questions in other subjects:


Health, 21.10.2021 03:00



Mathematics, 21.10.2021 03:00


Mathematics, 21.10.2021 03:00


Mathematics, 21.10.2021 03:00

Mathematics, 21.10.2021 03:00