
Chemistry, 02.07.2019 19:10, silviamgarcia
At 35°c, kc = 1.6 multiplied by10-5 for the following reaction
2 nocl(g) reverse reaction arrow 2 no(g)+ cl2(g)
calculate the concentrations of all species at equilibrium if
2.0 mol no and 1.0 mol of cl2 are placed in a 1.0 l flask

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, 4300402428
For the following reaction, 5.65 grams of oxygen gas are mixed with excess hydrochloric acid . assume that the percent yield of water is 86.4 %. hydrochloric acid(aq) + oxygen(g) water(l) + chlorine(g) what is the ideal yield of water ? grams what is the actual yield of water ? grams
Answers: 1

Chemistry, 21.06.2019 16:20, juandavidklingera553
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 23.06.2019 08:40, Riplilpeep
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1
Do you know the correct answer?
At 35°c, kc = 1.6 multiplied by10-5 for the following reaction
2 nocl(g) reverse reaction arro...
2 nocl(g) reverse reaction arro...
Questions in other subjects:

Biology, 22.11.2021 14:00


Biology, 22.11.2021 14:00


Social Studies, 22.11.2021 14:00

Mathematics, 22.11.2021 14:00

Biology, 22.11.2021 14:00



SAT, 22.11.2021 14:00


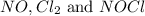 are, 0.05 M, 0.043 M and 0.975 M respectively.
are, 0.05 M, 0.043 M and 0.975 M respectively.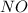 = 2 mole
= 2 mole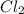 = 1 mole
= 1 mole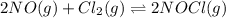
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0043/6110/56950.png)
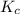 for reverse reaction =
for reverse reaction = 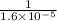
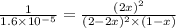
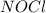 = x M = 0.975 M
= x M = 0.975 M



