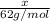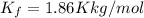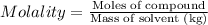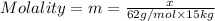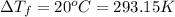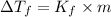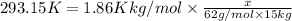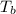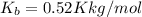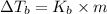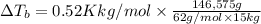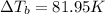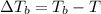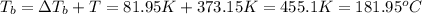Chemistry, 23.10.2019 10:00, jdmXdude3140
Ethylene glycol (c2h4(oh)2) , when dissolved in water, provides the standard ‘anti-freeze’ coolant for water-cooled engines. in order to depress the freezing point of water by 20 °c, how many grams of ethylene glycol would need to be dissolved in 15 kg of pure water? (the molal freezing point depression constant for water kf = 1.86 k mol-1 kg and the relevant atomic masses are: c = 12g, h = 1g and o = 16g.) note: ethylene glycol is an organic compound and does not break up or dissociate when it dissolves in water. hint: first calculate the molality (m) of the ethylene glycol solution.
what is the boiling point of this same solution at atmospheric pressure? (the molal boiling point elevation constant for water kb = 0.52 k mol -1 kg.)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
Do you know the correct answer?
Ethylene glycol (c2h4(oh)2) , when dissolved in water, provides the standard ‘anti-freeze’ coolant f...
Questions in other subjects:






Mathematics, 15.04.2020 03:18



Mathematics, 15.04.2020 03:18

Biology, 15.04.2020 03:18