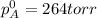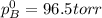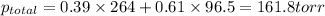Chemistry, 02.07.2019 04:10, NikkiZoeller
Liquid a has a vapor pressure of 264 torr at 20∘c, and liquid b has a vapor pressure of 96.5 torr at the same temperature. if 5.50 moles of liquid a and 8.50 moles of liquid b are combined to form an ideal solution, what is the total vapor pressure (in torr) above the solution at 20.0∘c?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 04:30, clairajogriggsk
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
Do you know the correct answer?
Liquid a has a vapor pressure of 264 torr at 20∘c, and liquid b has a vapor pressure of 96.5 torr at...
Questions in other subjects:

History, 07.07.2020 03:01


Mathematics, 07.07.2020 03:01

Mathematics, 07.07.2020 03:01

Physics, 07.07.2020 03:01



Mathematics, 07.07.2020 03:01

Mathematics, 07.07.2020 03:01

Health, 07.07.2020 03:01


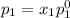 and
and 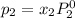
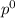 = pressure in the pure state
= pressure in the pure state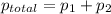
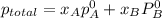
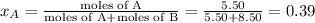 ,
, 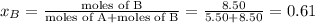 ,
,