
Chemistry, 02.07.2019 03:10, wedderman6049
You have 3.00 l of a 3.00 m solution of nacl(aq) called solution a. you also have 2.00 l of a 2.00 m solution of agno3(aq) called solution b. you mix these solutions together, making solution c. hint: agcl is a precipitate. calculate the concentrations (in m) of the following ions in solution c. no3-

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
Do you know the correct answer?
You have 3.00 l of a 3.00 m solution of nacl(aq) called solution a. you also have 2.00 l of a 2.00 m...
Questions in other subjects:

Social Studies, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

English, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Biology, 16.09.2020 02:01


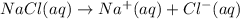
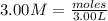
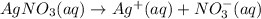
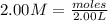
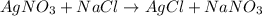
![[NO_{3}^-]=\frac{4 mol}{5 L}=0.8 mol/L](/tpl/images/0041/0466/43242.png)




