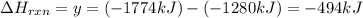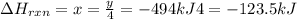Phosphorous pentachloride is used in the industrial preparation of many organic phosphorous compounds. equation i shows its preparation from pcl3 and cl2: (i) pcl3 (l) + cl2(g) pcl5(s) use equation ii and iii to calculate ∆hrxs of equation i: (ii) p4 (s) + 6 cl2 (g) 4 pcl3 (l) ∆h = 1280 kj (iii) p4 (s) + 10 cl2 (g) 4 pcl5 (s) ∆h = 1774 kj

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1


Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
Do you know the correct answer?
Phosphorous pentachloride is used in the industrial preparation of many organic phosphorous compound...
Questions in other subjects:

Mathematics, 06.09.2021 09:50

English, 06.09.2021 09:50



Mathematics, 06.09.2021 09:50

English, 06.09.2021 14:00

Mathematics, 06.09.2021 14:00


Biology, 06.09.2021 14:00

English, 06.09.2021 14:00


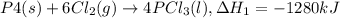 ..(1)
..(1)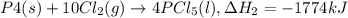 ..(2)
..(2)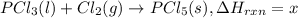 ...(3)
...(3)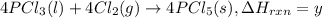
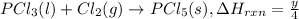 ...(3)
...(3)