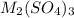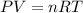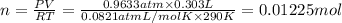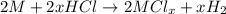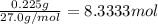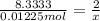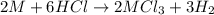Chemistry, 02.07.2019 00:10, naenae6775
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen (measured at 17°c and 741 mmhg) from an excess of hydrochloric acid. deduce from these data the corresponding equation and write formulas for the oxide and sulfate of m.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Do you know the correct answer?
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen...
Questions in other subjects:



Biology, 11.09.2021 14:00

History, 11.09.2021 14:00

Computers and Technology, 11.09.2021 14:00

History, 11.09.2021 14:00





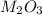 and sulfate of
and sulfate of