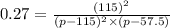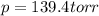Chemistry, 01.07.2019 23:30, NeverEndingCycle
Nitric oxide reacts with chlorine gas according to the reaction: 2 no( g) + cl2( g) ∆ 2 nocl( g) kp = 0.27 at 700 k a reaction mixture initially contains equal partial pressures of no and cl2. at equilibrium, the partial pressure of nocl is 115 torr. what were the initial partial pressures of no and cl2 ?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1


Chemistry, 23.06.2019 01:00, aliviadushane
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
Do you know the correct answer?
Nitric oxide reacts with chlorine gas according to the reaction: 2 no( g) + cl2( g) ∆ 2 nocl( g) kp...
Questions in other subjects:


History, 25.12.2020 04:20

Medicine, 25.12.2020 04:20


English, 25.12.2020 04:20

Computers and Technology, 25.12.2020 04:20


English, 25.12.2020 04:20


Business, 25.12.2020 04:20


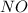 and
and 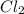 is 139.4 torr.
is 139.4 torr. 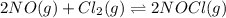
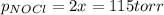
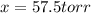
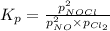
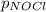 = 2x =115 torr
= 2x =115 torr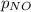 = (p-2x) = p-115 torr
= (p-2x) = p-115 torr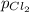 = (p-x)= p-57.5 torr
= (p-x)= p-57.5 torr