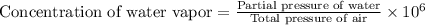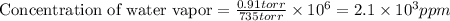Chemistry, 01.07.2019 23:30, itsyagirlbella
The concentration of water vapor in a sample of air that has a partial pressure of water of 0.91 torr and a total pressure of air of 735 torr is ppm. the concentration of water vapor in a sample of air that has a partial pressure of water of 0.91 torr and a total pressure of air of 735 torr is ppm. 0.81 0.12 8.1 ⋅ 10−4 1.2 1.2 ⋅ 103

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, petriajack8375
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Do you know the correct answer?
The concentration of water vapor in a sample of air that has a partial pressure of water of 0.91 tor...
Questions in other subjects:

Mathematics, 02.07.2019 12:40

Social Studies, 02.07.2019 12:40

Physics, 02.07.2019 12:40

Mathematics, 02.07.2019 12:40

Mathematics, 02.07.2019 12:40

History, 02.07.2019 12:40


History, 02.07.2019 12:40

History, 02.07.2019 12:40

Mathematics, 02.07.2019 12:40


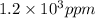
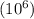 parts by the mass of the solution.
parts by the mass of the solution.