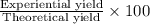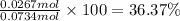Ammonia, nh3nh3 , can react with oxygen to form nitrogen gas and water. 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(l) 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(l) if 2.35 g2.35 g nh3nh3 reacts with 3.53 g3.53 g o2o2 and produces 0.650 l0.650 l n2n2 , at 295 k295 k and 1.01 bar1.01 bar , which reactant is limiting? o2(g)o2(g) nh3(aq)nh3(aq) calculate the percent yield of the reaction. percent yield:

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
Do you know the correct answer?
Ammonia, nh3nh3 , can react with oxygen to form nitrogen gas and water. 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(...
Questions in other subjects:

Mathematics, 30.01.2020 15:02

Mathematics, 30.01.2020 15:02

Biology, 30.01.2020 15:02




Mathematics, 30.01.2020 15:02




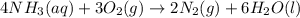
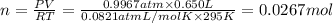
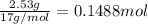
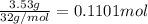
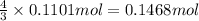 of ammonia.
of ammonia.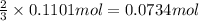 of nitrogen.
of nitrogen.