
Chemistry, 01.07.2019 22:40, ritahastie7533
The zero order reaction 2n2o→2n2+o2 has the reaction constant k is 6.28×10−3 moll s. if the initial concentration of n2o is 0.962 mol/l, what is the concentration of n2o after 10.0 seconds? your answer should have three significant figures (three decimal places).

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
Do you know the correct answer?
The zero order reaction 2n2o→2n2+o2 has the reaction constant k is 6.28×10−3 moll s. if the initial...
Questions in other subjects:

Mathematics, 10.07.2019 12:00



English, 10.07.2019 12:00

Mathematics, 10.07.2019 12:00

Health, 10.07.2019 12:00



History, 10.07.2019 12:00

Biology, 10.07.2019 12:00


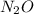 in three significant figures will be 0.899 mol/L.
in three significant figures will be 0.899 mol/L.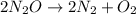
![k=\frac{1}{t}([A_o]-[A])](/tpl/images/0040/3296/5bd85.png)
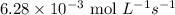
![[A_o]](/tpl/images/0040/3296/dc622.png) = initial concentration of the reactant = 0.962 mol/L
= initial concentration of the reactant = 0.962 mol/L![6.28\times 10^{-3}=\frac{1}{10}(0.962-[A])](/tpl/images/0040/3296/18cd6.png)
![[A]=0.899mol/L](/tpl/images/0040/3296/6a562.png)




