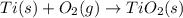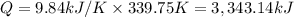The combustion of titanium with oxygen produces titanium dioxide:
ti (s) + o2 (g) → tio2 (s)<...

Chemistry, 01.07.2019 21:30, faithkristi
The combustion of titanium with oxygen produces titanium dioxide:
ti (s) + o2 (g) → tio2 (s)
when 2.060 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °c to 91.60 °c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.
ti = 47.867 amu
o2 = 31.9988 amu
tio2 = 79.8650 amu
report answer in scientific notation use en rather than x 10n

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Arts, 13.01.2021 01:00


Social Studies, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00



Mathematics, 13.01.2021 01:00



Mathematics, 13.01.2021 01:00


 .
.