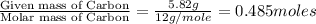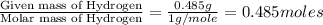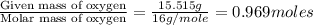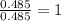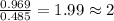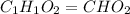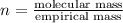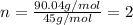Chemistry, 01.07.2019 21:20, robert7248
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis and 21.33 grams of co2 and 4.366 grams of h2o are produced. in a separate experiment, the molar mass is found to be 90.04 g/mol. determine the empirical formula and the molecular formula of the organic compound.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2


Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
Do you know the correct answer?
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis a...
Questions in other subjects:

Mathematics, 17.12.2020 21:10

Mathematics, 17.12.2020 21:10

Mathematics, 17.12.2020 21:10


Mathematics, 17.12.2020 21:10


English, 17.12.2020 21:10


Mathematics, 17.12.2020 21:10

Physics, 17.12.2020 21:10


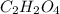
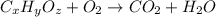
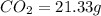
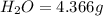
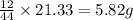 of carbon will be contained.
of carbon will be contained.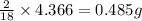 of hydrogen will be contained.
of hydrogen will be contained.