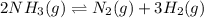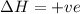Chemistry, 01.07.2019 20:30, ashley8057
The reaction below is allowed to come to equilibrium. after equilibrium is reached, the temperature of the container is raised by 50ºc. which statement below describes a change that will be observed as the system returns to an equilibrium state at the new temperature if δh is positive?
2 nh3(g) ⇄ n2(g) + 3 h2(g)
a. the concentration of ammonia, nh3, will increase.
b. the concentration of nitrogen gas, n2, will decrease.
c. the rate of the forward reaction will increase.
d. there will be no change; increasing the temperature will not change the position of equilibrium.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 17:20, banna01man
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
Do you know the correct answer?
The reaction below is allowed to come to equilibrium. after equilibrium is reached, the temperature...
Questions in other subjects:


English, 20.10.2020 01:01



Mathematics, 20.10.2020 01:01



English, 20.10.2020 01:01