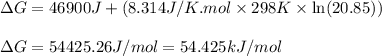Chemistry, 01.07.2019 19:20, elizavlsc4
Co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at 25 ∘c under these conditions: pco2pccl4pcocl2===0.140 atm0.185 atm0.735 atm δg∘f for co2(g) is −394.4kj/mol, δg∘f for ccl4(g) is −62.3kj/mol, and δg∘f for cocl2(g) is −204.9kj/mol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 23.06.2019 04:31, 1Angel2Got3Brains
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
Do you know the correct answer?
Co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at 25 ∘c under these conditions: pco2pccl4p...
Questions in other subjects:


History, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01





History, 21.10.2020 22:01



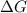 for the reaction is 54.425 kJ/mol
for the reaction is 54.425 kJ/mol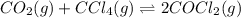
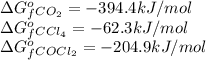
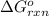 for the reaction, we use the equation:
for the reaction, we use the equation:![\Delta G^o_{rxn}=\sum [n\times \Delta G_f(product)]-\sum [n\times \Delta G_f(reactant)]](/tpl/images/0039/7802/1c133.png)
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(COCl_2)})]-[(1\times \Delta G^o_f_{(CO_2)})+(1\times \Delta G^o_f_{(CCl_4)})]](/tpl/images/0039/7802/08d2c.png)
![\Delta G^o_{rxn}=[(2\times (-204.9))-((1\times (-394.4))+(1\times (-62.3)))]\\\Delta G^o_{rxn}=46.9kJ=46900J](/tpl/images/0039/7802/b07a7.png)
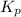 for the given reaction:
for the given reaction: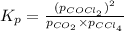
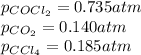
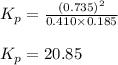
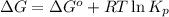
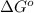 = Standard gibbs' free energy change of the reaction = 46900 J
= Standard gibbs' free energy change of the reaction = 46900 J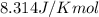
![25^oC=[25+273]K=298K](/tpl/images/0039/7802/df1f6.png)