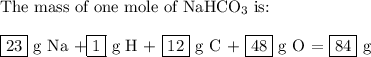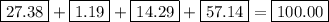Chemistry, 01.07.2019 00:10, lazerlemon500
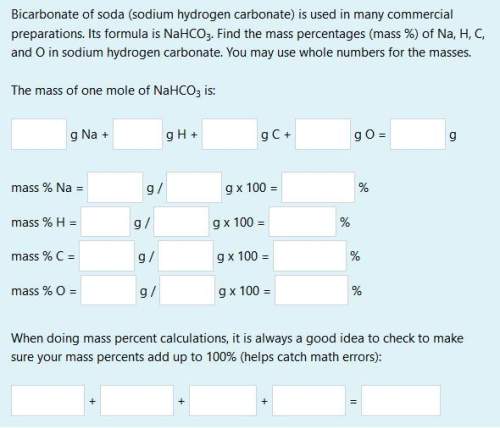
(need all boxes answered)
bicarbonate of soda (sodium hydrogen carbonate) is used in many commercial preparations. its formula is nahco3. find the mass percentages (mass %) of na, h, c, and o in sodium hydrogen carbonate. you may use whole numbers for the masses.
the mass of one mole of nahco3 is:

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
Do you know the correct answer?
(need all boxes answered)
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
Questions in other subjects:



Geography, 06.01.2022 05:40

Mathematics, 06.01.2022 05:40





SAT, 06.01.2022 05:50