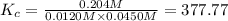Carbonyl chloride (cocl2), also called phosgene, was used in world war i as a poisonous gas. the equilibrium concentrations for the reaction between carbon monoxide and molecular chlorine to form carbonyl chloride at a certain temperature are [co] = 0.0210 m, [cl2] = 0.0450 m, and [cocl2] = 0.204 m. co(g) + cl2(g) ⇆ cocl2(g) calculate the equilibrium constant (kc).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 23.06.2019 10:00, Sariyahhall1
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
Do you know the correct answer?
Carbonyl chloride (cocl2), also called phosgene, was used in world war i as a poisonous gas. the equ...
Questions in other subjects:



Mathematics, 08.12.2020 21:30

English, 08.12.2020 21:30

History, 08.12.2020 21:30

English, 08.12.2020 21:30

History, 08.12.2020 21:30

History, 08.12.2020 21:30

Mathematics, 08.12.2020 21:30

Mathematics, 08.12.2020 21:30


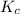
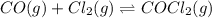
![[CO] = 0.0210 M](/tpl/images/0028/7944/23f4d.png)
![[Cl_2] = 0.0450 M](/tpl/images/0028/7944/558aa.png)
![[COCl_2] = 0.204 M](/tpl/images/0028/7944/1309b.png)
![K_c=\frac{[COCl_2]}{[CO][Cl_2]}](/tpl/images/0028/7944/36d91.png)