
Chemistry, 28.06.2019 22:30, netflixacc0107
Calculate the concentration of h3o⁺ in a solution that contains 5.5 × 10-5 m oh⁻ at 25°c. identify the solution as acidic, basic, or neutral. a) 1.8 × 10-10 m, basic b) 1.8 × 10-10 m, acidic c) 5.5 × 10-10 m, neutral d) 9.2 × 10-1 m, acidic e) 9.2 × 10-1 m, basic

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
Do you know the correct answer?
Calculate the concentration of h3o⁺ in a solution that contains 5.5 × 10-5 m oh⁻ at 25°c. identify t...
Questions in other subjects:






Mathematics, 11.02.2020 20:00





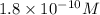 , basic.
, basic.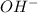 ion =
ion = 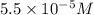
![pOH=-\log [OH^-]](/tpl/images/0028/7490/1fac1.png)
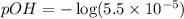
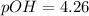
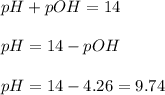
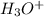 concentration.
concentration.![pH=-\log [H_3O^+]](/tpl/images/0028/7490/841e8.png)
![9.74=-\log [H_3O^+]](/tpl/images/0028/7490/7438d.png)
![[H_3O^+]=1.8\times 10^{-10}M](/tpl/images/0028/7490/bf6a6.png)





