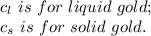Chemistry, 28.06.2019 18:30, ilizzy1224
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg of liquid gold at an initial temperature at 1336k. the solid and liquid other. when the two reach thermal equilibrium will the mixture be entirely solid, or will they be in a mixed solid/liquid phase? explain how you know. draw two separate temp. vs. energy added diagrams to you answer this question. can only exchange heat with each

Answers: 3
Similar questions


Chemistry, 26.07.2019 06:10, mari048
Answers: 2

Physics, 22.08.2019 03:30, mfreeman1096
Answers: 2

Physics, 20.09.2019 02:10, juansantos7b
Answers: 3
Do you know the correct answer?
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg...
Questions in other subjects:

Arts, 24.02.2021 16:50


Business, 24.02.2021 16:50




History, 24.02.2021 16:50