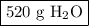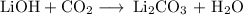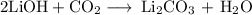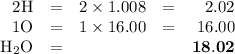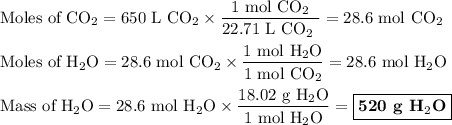Chemistry, 28.06.2019 04:10, lilymessina94
Answer the following question: in a space shuttle, the co2 that the crew exhales is removed from the air by a reaction within canisters of lithium hydroxide. on average, each astronaut exhales about 650 l of co2 daily. what mass of water will be produced
when this amount reacts with lioh? the other product of the reaction is
li2co3. when answering this question include the following:
have both the unbalanced and balanced chemical equations.
explain how to find the molar mass of the compounds.
explain how the balanced chemical equation is used to find the ratio of moles (hint: step 3 in the video).
explain how many significant figures your answer needs to have.
the numerical answer

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
Do you know the correct answer?
Answer the following question: in a space shuttle, the co2 that the crew exhales is removed from th...
Questions in other subjects:


Mathematics, 04.12.2021 16:30


World Languages, 04.12.2021 16:30




Geography, 04.12.2021 16:40


English, 04.12.2021 16:40