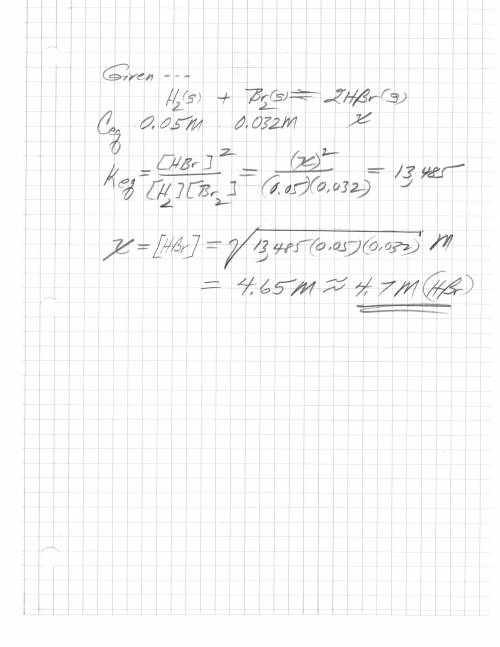
H2 (g) + br2 (g) < => 2 hbr (g)
the equilibrium constant is 13485. at equilibrium...

Chemistry, 23.11.2019 20:31, abronxtale02
H2 (g) + br2 (g) < => 2 hbr (g)
the equilibrium constant is 13485. at equilibrium the h2 concentration is 0.05 m, while the br2 concentration is 0.023 m. calculate the hbr concentration at equilibrium, to 1 decimal. be careful with the units.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, S4NCHEZ28
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Geography, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00