
Chemistry, 29.01.2020 19:02, Pizzapegasus1
Calculate the enthalpy change associated with the conversion of 25.0 grams of ice at -4.00 °c to water vapor at 110.0 °c. the specific heats of ice, water, and steam are 2.09 j/g-k, 4.18 j/g-k, and 1.84 j/g-k, respectively. for , δhfus = 6.01 kj/mol and δhvap = 40.67 kj/mol.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
Do you know the correct answer?
Calculate the enthalpy change associated with the conversion of 25.0 grams of ice at -4.00 °c to wat...
Questions in other subjects:

Mathematics, 07.07.2019 09:00

Mathematics, 07.07.2019 09:00


History, 07.07.2019 09:00





Mathematics, 07.07.2019 09:00

Mathematics, 07.07.2019 09:00



![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0482/9975/e4ef0.png)
 = enthalpy change = ?
= enthalpy change = ? = specific heat of solid water = 2.09 J/gk
= specific heat of solid water = 2.09 J/gk = specific heat of liquid water = 4.18 J/gk
= specific heat of liquid water = 4.18 J/gk = specific heat of liquid water = 1.84 J/gk
= specific heat of liquid water = 1.84 J/gk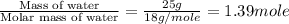
 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[25g\times 4.18J/gK\times (273-277)k]+1.39mole\times 6010J/mole+[25g\times 2.09J/gK\times (373-273)k]+1.39mole\times 40670J/mole+[25g\times 1.84J/gK\times (383-373)k]](/tpl/images/0482/9975/b0606.png)
 (1 KJ = 1000 J)
(1 KJ = 1000 J)



