Chemistry, 27.06.2019 10:10, Chatoloko231
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how many moles are present in this sample?
when answering the question, include the following:
state how to find the molar mass for the hydrocarbon.
state how you know if you need to multiply or divide by the molar mass.
give the correct number of significant figures and explain why the answer has that many significant figures.

Answers: 3
Other questions on the subject: Chemistry



Do you know the correct answer?
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how m...
Questions in other subjects:


Mathematics, 07.09.2020 18:01


Mathematics, 07.09.2020 18:01

English, 07.09.2020 18:01

Geography, 07.09.2020 18:01

Geography, 07.09.2020 18:01


History, 07.09.2020 18:01


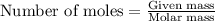
![[(2\times 12)+(6\times 1)]=30g/mol](/tpl/images/0022/9571/19171.png)