
Chemistry, 27.06.2019 09:10, alleshia2007
If 58.67g of mercuric oxide were completely decomposed to generate 54.34 g of mercury how many grams of oxygen should have been produced

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
Do you know the correct answer?
If 58.67g of mercuric oxide were completely decomposed to generate 54.34 g of mercury how many grams...
Questions in other subjects:





Mathematics, 01.08.2020 20:01






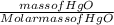
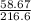 = 0.271mol
= 0.271mol



