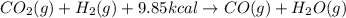Chemistry, 26.06.2019 20:20, Santos7446
When co2(g) reacts with h2(g) to form co(g) and h2o(g) , 9.85 kcal of energy are absorbed for each mole of co2(g) that reacts. write a balanced equation for the reaction with an energy term in kcal as part of the equation.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, thickness7699
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 07:30, eburnhisel2023
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
Do you know the correct answer?
When co2(g) reacts with h2(g) to form co(g) and h2o(g) , 9.85 kcal of energy are absorbed for each m...
Questions in other subjects:

English, 22.01.2022 14:00

Chemistry, 22.01.2022 14:00

Mathematics, 22.01.2022 14:00

Mathematics, 22.01.2022 14:00

Chemistry, 22.01.2022 14:00



Mathematics, 22.01.2022 14:00


English, 22.01.2022 14:00