Chemistry, 05.02.2020 00:02, mercydiaz84
(a) write the balanced neutralization reaction that occurs between h2so4 and koh in aqueous solution. phases are optional. (b) suppose 0.750 l of 0.480 m h2so4 is mixed with 0.700 l of 0.290 m koh. what concentration of sulfuric acid remains after neutralization?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3


Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
Do you know the correct answer?
(a) write the balanced neutralization reaction that occurs between h2so4 and koh in aqueous solution...
Questions in other subjects:

Mathematics, 21.05.2020 09:59




Physics, 21.05.2020 09:59


Mathematics, 21.05.2020 09:59

Mathematics, 21.05.2020 09:59

Mathematics, 21.05.2020 09:59

Mathematics, 21.05.2020 09:59



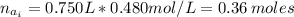
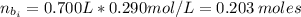




 : is the total volume = (0.750 + 0.700) L = 1.45 L
: is the total volume = (0.750 + 0.700) L = 1.45 L