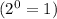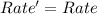Chemistry, 26.06.2019 06:10, katiem7608
Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and second order in c. by what factor does the reaction rate change if [a] is doubled (and the other reactant concentrations are held constant)?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
Do you know the correct answer?
Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and s...
Questions in other subjects:

Chemistry, 28.06.2020 01:01

Mathematics, 28.06.2020 01:01

Biology, 28.06.2020 01:01

Mathematics, 28.06.2020 01:01

History, 28.06.2020 01:01


Mathematics, 28.06.2020 01:01

Mathematics, 28.06.2020 01:01


Mathematics, 28.06.2020 01:01



![Rate=k[A]^x[B]^y[C]^z](/tpl/images/0018/4732/b91f4.png)
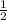
![Rate=k[A]^0[B]^{\frac{1}{2}}[C]^2](/tpl/images/0018/4732/f55a3.png)
![Rate'=k[2A]^0[B]^{\frac{1}{2}}[C]^2](/tpl/images/0018/4732/4c93e.png)
![Rate'=k[2]^0[A]^0[B]^{\frac{1}{2}}[C]^2](/tpl/images/0018/4732/36c04.png)
![Rate'=k[A]^0[B]^{\frac{1}{2}}[C]^2](/tpl/images/0018/4732/23a75.png)