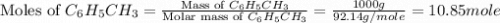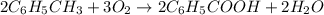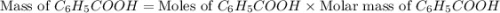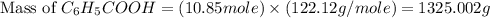Chemistry, 26.06.2019 04:10, samsavage4073
Toluene, c6h5ch3, is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h, which is used to prepare the food preservative sodium benzoate, c6h5co2na. what is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
Do you know the correct answer?
Toluene, c6h5ch3, is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h...
Questions in other subjects:



History, 04.02.2020 03:51

English, 04.02.2020 03:51

Mathematics, 04.02.2020 03:51


Mathematics, 04.02.2020 03:51

Mathematics, 04.02.2020 03:51


Mathematics, 04.02.2020 03:51


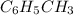 = 1 Kg = 1000 g
= 1 Kg = 1000 g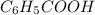 = 122.12 g/mole
= 122.12 g/mole