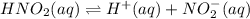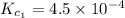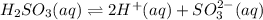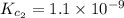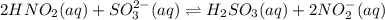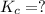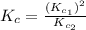Given the equilibrium constants for the following two reactions in aqueous solution at 25 ∘c hno2(aq)h2so3(aq)⇌⇌h+(aq) + no2−(aq)2h+(aq) + so32−(aq)kc = 4.5 × 10−4kc = 1.1 × 10−9 what is the value of kc for the reaction 2hno2(aq) + so32−(aq)⇌h2so3(aq) + 2no2−(aq)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, anamaliiow
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 21.06.2019 19:30, jetblackcap
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1
Do you know the correct answer?
Given the equilibrium constants for the following two reactions in aqueous solution at 25 ∘c hno2(aq...
Questions in other subjects:



History, 04.10.2019 22:10



Social Studies, 04.10.2019 22:10

Social Studies, 04.10.2019 22:10




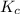 for the final reaction is, 184.09
for the final reaction is, 184.09