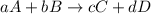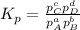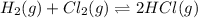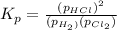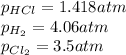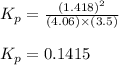Chemistry, 26.06.2019 03:20, elopezhilario6339
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibrium. the equilibrium pressure of hcl is found to be 1.418 atm. calculate kp for the reaction at this temperature. h2(g) + cl2(g) < => 2 hcl(g)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
Do you know the correct answer?
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibri...
Questions in other subjects:

Mathematics, 22.09.2019 03:00

History, 22.09.2019 03:00






Mathematics, 22.09.2019 03:00


Health, 22.09.2019 03:00


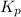 for the given chemical reaction is 0.1415
for the given chemical reaction is 0.1415