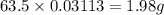Chemistry, 23.01.2020 04:31, caitlynnstokes
In an electroplating process, copper (ionic charge +2e, atomic weight 63.6 g/mol) is deposited using a current of 10.0 a. what mass of copper is deposited in 10.0 minutes? avogadro's number is 6.022 × 1023 molecules/mol and e = 1.60 × 10-19 c.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 10:00, anonymous176
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3
Do you know the correct answer?
In an electroplating process, copper (ionic charge +2e, atomic weight 63.6 g/mol) is deposited using...
Questions in other subjects:

History, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Social Studies, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

English, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Arts, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01


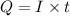
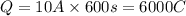
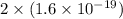
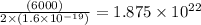 atoms
atoms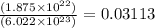 moles
moles