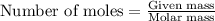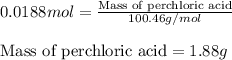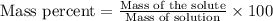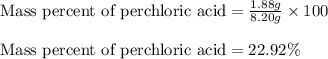Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
Do you know the correct answer?
A8.20 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. if...
Questions in other subjects:

Mathematics, 26.02.2021 21:00

Biology, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00


Biology, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00


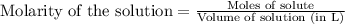
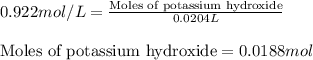
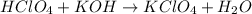
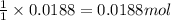 of perchloric acid.
of perchloric acid.