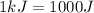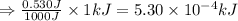Chemistry, 31.01.2020 22:02, ComicSans01
Suppose you are investigating the reaction: m(s) + 2 hcl(aq) → mcl2(aq) + h2(g). you weigh out a 0.295 gram piece of metal and combine it with 65 ml of 1.00 m hcl in a coffee-cup calorimeter. if the molar mass of the metal is 57.78 g/mol, and you measure that the reaction absorbed 104 j of heat, what is the enthalpy of this reaction in kj per mole of limiting reactant? enter your answer numerically to three significant figures in units of kj/mol.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1
Do you know the correct answer?
Suppose you are investigating the reaction: m(s) + 2 hcl(aq) → mcl2(aq) + h2(g). you weigh out a 0....
Questions in other subjects:

Biology, 11.12.2019 19:31



History, 11.12.2019 19:31


Mathematics, 11.12.2019 19:31




English, 11.12.2019 19:31


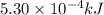
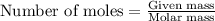 ....(1)
....(1)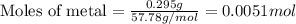
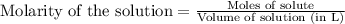
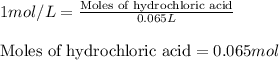
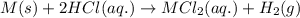
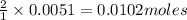 of hydrochloric acid.
of hydrochloric acid.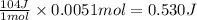 of heat.
of heat.