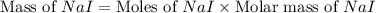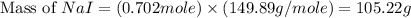Chemistry, 15.11.2019 14:31, ejfleck3655
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solution containing sodium iodide. 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) how many grams of sodium iodide, nai, nai, must be used to produce 89.1 g89.1 g of iodine, i2? i2? mass: g nai

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 04:30, Har13526574
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
Do you know the correct answer?
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solu...
Questions in other subjects:



Physics, 15.08.2021 20:10

Business, 15.08.2021 20:10




Mathematics, 15.08.2021 20:10

Mathematics, 15.08.2021 20:10


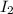 = 89.1 g
= 89.1 g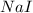 = 149.89 g/mole
= 149.89 g/mole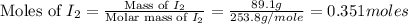
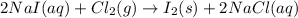
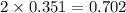 moles of
moles of