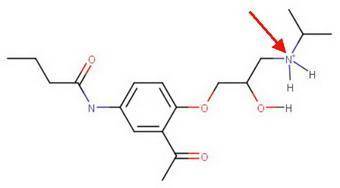
Many drugs are sold as their hydrochloric salts (r2nh2 cl−), formed by reaction of an amine (r2nh) with hcl. a. draw the major organic product formed from the formation of acebutolol with hcl. acebutolol is a β blocker used to treat high blood pressure. omit any inorganic counterions. b. discuss the solubility of acebutlol and its hydrochloride salt in water. c. offer a reason as to why the drug is marketed as a hydrochloride salt rather than a neutral amine.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 05:00, contrerasdaisy100
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g 89% just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g show your calculations below. analysis questions 1. based on taste observations only, which ingredients were in excess in the lemonade samples in activity one? in activity one the excess substances for each sample were the water and sugar. 2. based on the data in activity two, which excess ingredients are affecting the taste of the lemonade in the sample batch? 3. what can just lemons, inc. do during production to reduce the amount of excess ingredients and improve the taste of their lemonade? 4. try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. 5. during factory inspection, just lemons, inc. discovered that a water valve to the lemonade mixing station was not functioning. once they repair it, more water will enter the mixing station. from what you know about the limiting and excess ingredients for current lemonade production, what advice would you give engineers about the upcoming increase in water?
Answers: 3

Chemistry, 23.06.2019 08:00, colbygreen6189
Identify the decay mode particle emitted from the th 234
Answers: 1
Do you know the correct answer?
Many drugs are sold as their hydrochloric salts (r2nh2 cl−), formed by reaction of an amine (r2nh) w...
Questions in other subjects:


Mathematics, 23.10.2020 23:50



Chemistry, 23.10.2020 23:50

Biology, 23.10.2020 23:50

Mathematics, 23.10.2020 23:50

Chemistry, 23.10.2020 23:50