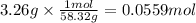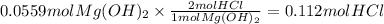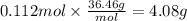Chemistry, 16.10.2019 23:30, wiljoystoltz253
Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily hcl, according to the reaction mg(oh)2(aq)+2hcl(aq)→2h2o(l)+mgcl2( aq) what mass of hcl, in grams, is neutralized by a dose of milk of magnesia containing 3.26 g of mg(oh)2? express the mass in grams to three significant figures.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
Do you know the correct answer?
Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily...
Questions in other subjects:

Chemistry, 20.04.2021 20:00




Mathematics, 20.04.2021 20:00




Mathematics, 20.04.2021 20:00

Mathematics, 20.04.2021 20:00