
Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
Do you know the correct answer?
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has...
Questions in other subjects:

Chemistry, 14.01.2021 06:50

Mathematics, 14.01.2021 06:50


Mathematics, 14.01.2021 06:50


Biology, 14.01.2021 06:50

Advanced Placement (AP), 14.01.2021 06:50

Chemistry, 14.01.2021 06:50


Mathematics, 14.01.2021 06:50


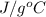
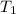 =
= 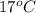 , and
, and 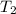 =
= 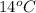
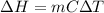
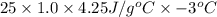 (as density =
(as density = 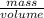 )
)



