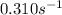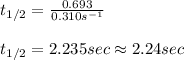Chemistry, 19.11.2019 10:31, PlaneGamer5678
The initial concentration of a in the first-order reaction 4a→4b+c is 0.933 mol l−1. given that the rate constant is 0.310 s−1, what is the half-life of the reaction in seconds? remember to use correct significant figures in your answer (round your answer to the nearest hundredth). do not include units in your response.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1


Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
Do you know the correct answer?
The initial concentration of a in the first-order reaction 4a→4b+c is 0.933 mol l−1. given that the...
Questions in other subjects:




Biology, 26.11.2020 01:40

History, 26.11.2020 01:40

Computers and Technology, 26.11.2020 01:40


Mathematics, 26.11.2020 01:40


Mathematics, 26.11.2020 01:40



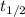 = half-life of the reaction
= half-life of the reaction