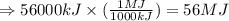Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 06:00, asalimanoucha2v
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
Do you know the correct answer?
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the...
Questions in other subjects:


Mathematics, 31.01.2020 04:50


History, 31.01.2020 04:50



History, 31.01.2020 04:50

Chemistry, 31.01.2020 04:50

Mathematics, 31.01.2020 04:50

Mathematics, 31.01.2020 04:50


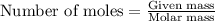
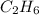 = 12 kg = 12000 g (Conversion factor: 1 kg = 1000 g)
= 12 kg = 12000 g (Conversion factor: 1 kg = 1000 g)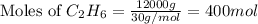
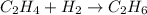
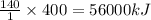 of heat.
of heat.