
Chemistry, 23.06.2019 03:40, ElegantEmerald
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1


Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
Do you know the correct answer?
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh)....
Questions in other subjects:

Mathematics, 14.09.2020 16:01

Social Studies, 14.09.2020 16:01

Geography, 14.09.2020 16:01

Social Studies, 14.09.2020 16:01

English, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

English, 14.09.2020 16:01


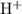 be
be 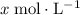 . Note that
. Note that 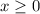 .
.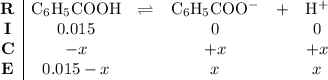
![\displaystyle \frac{[\mathrm{C_6H_5COO^{-}}]\cdot [\mathrm{H^{+}}]}{[\mathrm{C_6H_5COOH}]} = \mathrm{pK}_{a}](/tpl/images/0006/5046/265ec.png) .
.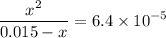 .
. :
: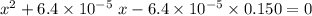 .
.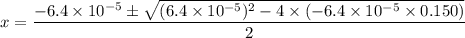 .
.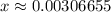 .
.![\rm [H^{+}] = 0.00306655\; mol\cdot L^{-1}](/tpl/images/0006/5046/db736.png) .
.![\displaystyle \mathrm{pH} = -\log_{10}{[\mathrm{H^{+}}]} = 2.513](/tpl/images/0006/5046/141cf.png) .
.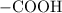 . Benzoic acid is thus a monoprotic acid. Each mole of the acid will react with only one mole of
. Benzoic acid is thus a monoprotic acid. Each mole of the acid will react with only one mole of 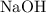 . The 100 mL solution initially contains
. The 100 mL solution initially contains 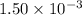 moles of benzoic acid. The
moles of benzoic acid. The 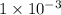 moles of
moles of 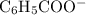 (from the salt
(from the salt 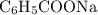 ) and
) and 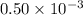 moles of
moles of 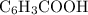 .
.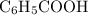 and the conjugate base of the acid
and the conjugate base of the acid 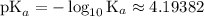 for benzoic acid.
for benzoic acid.![\begin{aligned}\mathrm{pH} &= \mathrm{pK}_{a} + \log{\frac{{[\text{Conjugate Base}]}}{[\text{Weak Acid}]}} \\ &= \mathrm{pK}_{a} + \log{\frac{{[\mathrm{C_6H_5COO^{-}}]}}{[\mathrm{C_6H_5COOH}]}}\\ &= 4.19382 + \log{\frac{0.01}{0.005}}\\ &\approx 4.495 \end{aligned}](/tpl/images/0006/5046/20b2b.png) .
.



