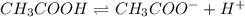Chemistry, 24.06.2019 02:20, connorwbrown07
For the reaction ch3cooh > ch3coo- + h+, which of the following statements is true? a.) ch3cooh is a bronsted lowry base. b.) ch3coo- is a bronsted lowry base. c.) ch3coo- is a conjugate base. d.) ch3coo- is an arrhenius base.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Do you know the correct answer?
For the reaction ch3cooh > ch3coo- + h+, which of the following statements is true? a.) ch3cooh...
Questions in other subjects:


Physics, 13.11.2020 03:40




History, 13.11.2020 03:40

Social Studies, 13.11.2020 03:40


Mathematics, 13.11.2020 03:40


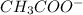 is a Bronsted Lowry base.
is a Bronsted Lowry base.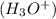 or hydrogen ion
or hydrogen ion 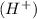 and a bases is a substance that ionizes in the water to give hydroxide ion
and a bases is a substance that ionizes in the water to give hydroxide ion 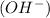 .
.