

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2


Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
Do you know the correct answer?
Calculate the energy in calories required to produce, from neutral he atoms,1 mole of he+ ions and h...
Questions in other subjects:

Biology, 11.05.2021 02:30


English, 11.05.2021 02:30

History, 11.05.2021 02:30






Mathematics, 11.05.2021 02:30


![\Delta E = R_{H}[\frac{1}{n^{2}_{i}} - \frac{1}{n^{2}_{f}}]](/tpl/images/0010/5843/a1c6b.png)
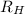 = Reydberg's constant =
= Reydberg's constant = 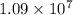 per meter
per meter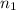 = 1 and
= 1 and 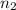 =
= 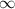
![1.09 \times 10^{7}[\frac{1}{(1)^{2}} - \frac{1}{\infty}}]](/tpl/images/0010/5843/d56c8.png) J
J ![1.09 \times 10^{7}[1 - 0}]](/tpl/images/0010/5843/bc35e.png) J
J ![1.09 \times 10^{7}]](/tpl/images/0010/5843/17892.png) J
J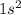 . Thus, energy required to produce
. Thus, energy required to produce 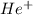 and
and 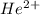 will be the same.
will be the same.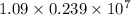 J
J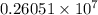 cal
cal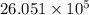 cal
cal



