
Chemistry, 25.06.2019 19:50, summerdooleyu
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is produced. b. co2 concentration increases. c. the equilibrium is pushed in the direction of reactants. d. nothing
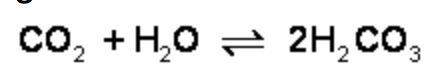

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 12:00, snikergrace
Which of the following statements is true? a. most heat energy is easily recovered and used for useful actions. b. friction causes molecules to vibrate more slowly. burning air and gasoline in an c. engine changes chemical energy into mechanical energy. it is impossible to d. change mechanical energy into mechanical energy.
Answers: 1
Do you know the correct answer?
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is pro...
Questions in other subjects:



Social Studies, 08.12.2020 01:40

English, 08.12.2020 01:40



Mathematics, 08.12.2020 01:40

English, 08.12.2020 01:40







