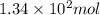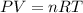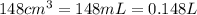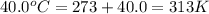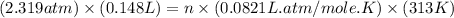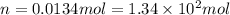Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, FloweyFlower
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
Do you know the correct answer?
At 40.0°c, the pressure inside a nitrogen-filled tennis ball with a volume of 148 cm is 235 kpa. how...
Questions in other subjects:


Mathematics, 17.10.2020 20:01





Mathematics, 17.10.2020 20:01

Social Studies, 17.10.2020 20:01


Biology, 17.10.2020 20:01